Despite its moment measurement, a solitary mobile contains billions of molecules that bustle all over and bind to a person an additional, carrying out important capabilities. The human genome encodes about 20,000 proteins, most of which interact with partner proteins to mediate upwards of 400,000 unique interactions. These associates really do not just latch on to a person another haphazardly they only bind to pretty particular companions that they ought to understand inside the crowded cell. If they build the mistaken pairings — or even the right pairings at the incorrect spot or completely wrong time — cancer or other illnesses can ensue. Researchers are really hard at get the job done investigating these protein-protein associations, in get to comprehend how they perform, and probably produce prescription drugs that disrupt or mimic them to take care of condition.
The average human protein is composed of roughly 400 constructing blocks termed amino acids, which are strung together and folded into a sophisticated 3D construction. Within just this long string of constructing blocks, some proteins consist of stretches of four to six amino acids identified as limited linear motifs (SLiMs), which mediate protein-protein interactions. Even with their simplicity and tiny size, SLiMs and their binding companions facilitate essential cellular processes. However, it is been historically tough to devise experiments to probe how SLiMs realize their precise binding companions.
To handle this trouble, a team led by Theresa Hwang PhD ’21 made a screening approach to understand how SLiMs selectively bind to specified proteins, and even distinguish involving individuals with identical buildings. Working with the in-depth info they gleaned from studying these interactions, the researchers produced their very own artificial molecule able of binding incredibly tightly to a protein termed ENAH, which is implicated in most cancers metastasis. The crew shared their results in a pair of eLife research, one particular published on Dec. 2, 2021, and the other posted Jan. 25.
“The means to take a look at hundreds of thousands of probable SLiMs for binding presents a effective instrument to examine why proteins favor distinct Slender partners in excess of some others,” suggests Amy Keating, professor of biology and organic engineering and the senior creator on the two research. “As we get an knowing of the methods that a protein employs to select its partners, we can use these in protein design and style to make our personal binders to modulate protein purpose for study or therapeutic purposes.”
Most present screens for SLiMs just pick for small, limited binders, even though neglecting SLiMs that do not grip their partner proteins very as strongly. To survey SLiMs with a extensive array of binding affinities, Keating, Hwang, and their colleagues made their have screen identified as MassTitr.
The researchers also suspected that the amino acids on possibly facet of the SLiM’s main 4-to-six amino acid sequence may possibly enjoy an underappreciated part in binding. To check their theory, they made use of MassTitr to screen the human proteome in for a longer period chunks comprised of 36 amino acids, in get to see which “extended” SLiMs would affiliate with the protein ENAH.
ENAH, sometimes referred to as Mena, helps cells to shift. This ability to migrate is important for healthy cells, but cancer cells can co-decide it to spread. Researchers have discovered that decreasing the amount of ENAH decreases the cancer cell’s ability to invade other tissues — suggesting that formulating medicine to disrupt this protein and its interactions could treat cancer.
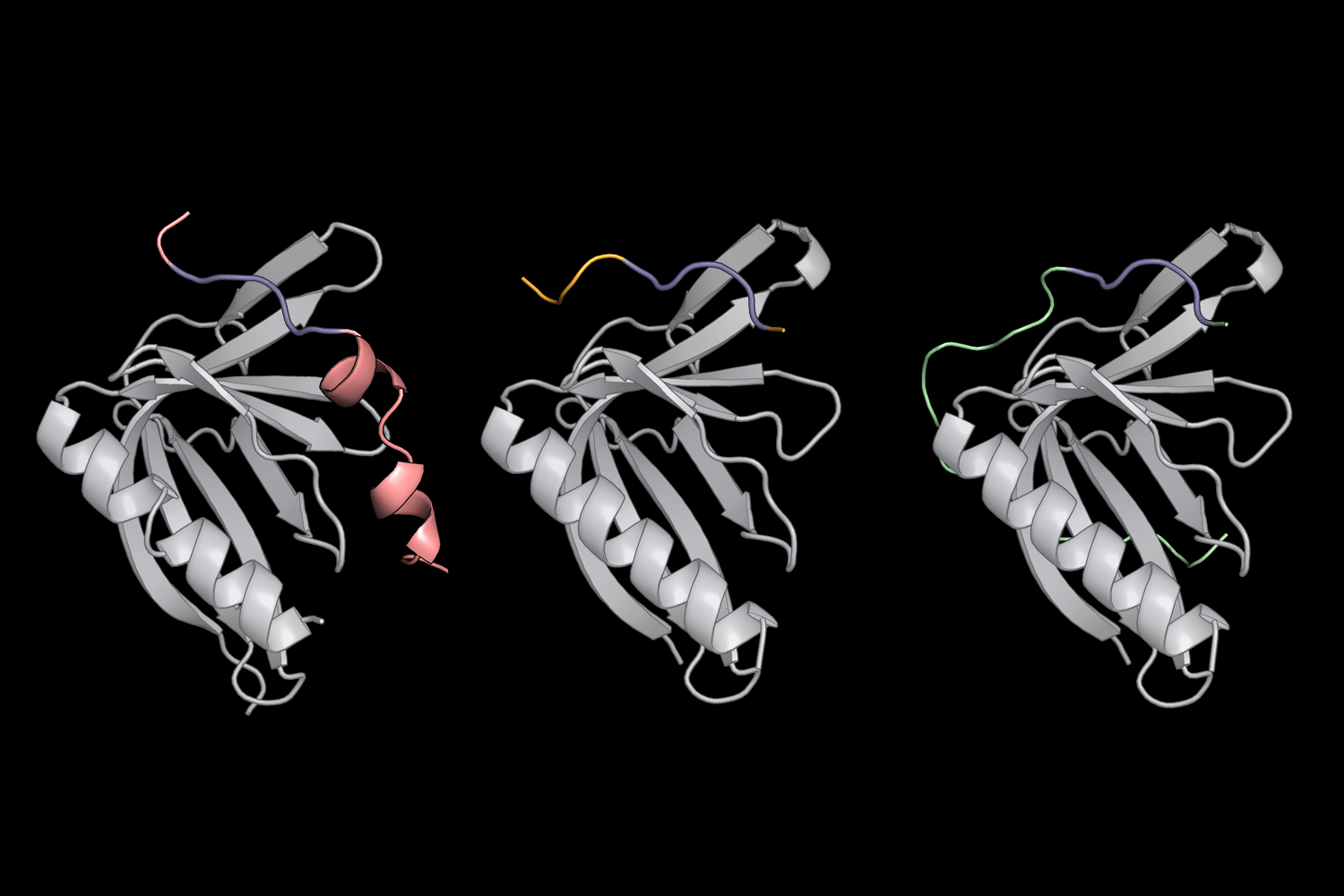
Thanks to MassTitr, the staff recognized 33 Slim-that contains proteins that bound to ENAH — 19 of which are possibly novel binding partners. They also discovered 3 unique patterns of amino acids flanking core Slim sequences that assisted the SLiMs bind even tighter to ENAH. Of these prolonged SLiMs, just one observed in a protein known as PCARE certain to ENAH with the highest known affinity of any Slender to day.
Upcoming, the researchers put together a pc method named dTERMen with X-ray crystallography in get have an understanding of how and why PCARE binds to ENAH in excess of ENAH’s two virtually equivalent sister proteins (VASP and EVL). Hwang and her colleagues observed that the amino acids flanking PCARE’s core Slender induced ENAH to alter shape slightly when the two built contact, permitting the binding web-sites to latch on to 1 yet another. VASP and EVL, by distinction, could not undergo this structural improve, so the PCARE Trim did not bind to both of them as tightly.
Motivated by this unique interaction, Hwang built her possess protein that sure to ENAH with unprecedented affinity and specificity. “It was exciting that we had been capable to come up with these a particular binder,” she claims. “This function lays the basis for creating artificial molecules with the probable to disrupt protein-protein interactions that lead to condition — or to assist researchers master a lot more about ENAH and other Slim-binding proteins.”
Ylva Ivarsson, a professor of biochemistry at Uppsala College who was not involved with the research, says that being familiar with how proteins locate their binding associates is a concern of elementary great importance to mobile purpose and regulation. The two eLife experiments, she describes, clearly show that extended SLiMs participate in an underappreciated purpose in deciding the affinity and specificity of these binding interactions.
“The studies drop gentle on the concept that context matters, and give a screening strategy for a range of context-dependent binding interactions,” she claims. “Hwang and co-authors have produced valuable instruments for dissecting the cellular purpose of proteins and their binding associates. Their approach could even inspire ENAH-precise inhibitors for therapeutic uses.”
Hwang’s most important takeaway from the task is that things are not normally as they look: even quick, basic protein segments can engage in complicated roles in the mobile. As she places it: “We really should really respect SLiMs additional.”
